The Crucial Role of Raw Materials in Innovative Medical Devices
- techfitds
- Aug 14, 2023
- 2 min read
At TECHFIT Digital Surgery, our unwavering commitment is centered on enhancing patient well-being through the provision of impeccable medical tools. Our guiding principle, "patients come first," fuels our relentless pursuit of delivering optimal solutions. Central to the realm of medical devices is the pivotal aspect of selecting the most appropriate raw materials. This choice significantly influences the safety, functionality, and efficacy of the final product post-manufacturing.
The art of choosing the right raw material transcends mere science. It's an orchestration of properties that harmonize seamlessly: biocompatibility, mechanical resilience, sterilization feasibility, and application suitability. Imagine a symphony where each instrument plays its role to perfection. Take, for instance, PEEK (Polyether Ether Ketone), a star player in our repertoire. Renowned for its biocompatibility, PEEK averts toxic or allergic reactions in patients, while its sterilizability further accentuates its value. Moreover, its mechanical properties, akin to the human body's elasticity, mitigate the risk of implant-induced osteolysis and bone resorption, a result of stress shielding [1], [2].
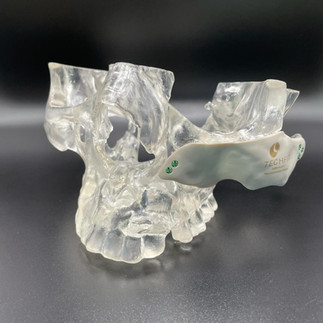
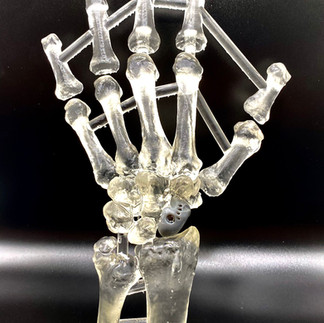
Another prominent example is the application of pure titanium in TECHFIT's cranial implants, surgical guides, and maxillofacial and orthopedic plates. Titanium's extensive popularity over six decades in dental and orthopedic implants can be attributed to its remarkable attributes such as strength, durability, corrosion resistance, and superior biocompatibility [3].
Yet, the tale doesn't end with scientific validation. Enter the realm of standards that define a material's attributes based on its destined role. TECHFIT diligently adheres to ASTM F2026-17 and ASTM F67 standards for PEEK and titanium materials respectively, pertaining to implantable devices.
TECHFIT's dedication transcends the choice of premium raw materials; the intricacies of the manufacturing process are equally pivotal. Our quest for excellence continuously evolves, with manufacturing processes honed to perfection. Continual validation and verification of manufacturing processes underscore our commitment to patient safety and product performance. Rigorous biological and performance tests are integral components of this quality assurance endeavor.
The scope of our biological tests encompasses:
Cleaning Tests: Ensuring meticulous removal of all residues from the manufacturing process.
Biological Tests: Safeguarding against adverse reactions during patient-device interactions.
Chemical Tests: Peering into the organic and inorganic compounds lingering within the final device.
Likewise, pivotal performance evaluations entail:
Mechanical Strength: Bending, tension, and compression tests ensure the device's robustness aligns with its purpose.
Compatibility Test: Ensuring seamless synergy with other TECHFIT devices, orchestrating intricate procedures with precision.
Dimensional Validation: Mirroring the digital design with tangible perfection. Verification of congruence between CAD-designed devices and their manufactured counterparts.
In essence, TECHFIT's dedication transcends excellence. We don't just offer devices; we deliver a promise—crafted from superior raw materials, honed by meticulous processes, and resonating with biocompatibility. Our mission echoes clarity: to embolden surgeon's trust and confidence in the materials and devices entrusted with the patients' care. Join us in shaping a healthier tomorrow—one TECHFIT device at a time.
References
[1] Sarfraz, S., Mäntynen, P., Laurila, M., Rossi, S., Leikola, J., Kaakinen, M., Suojanen, J., & Reunanen, J. (2022). Comparison of Titanium and PEEK Medical Plastic Implant Materials for Their Bacterial Biofilm Formation Properties. Polymers, 14(18), 3862. https://doi.org/10.3390/polym14183862
[2] Stratton-Powell, A.A., Pasko, K.M., Brockett, C.L. et al. The Biologic Response to Polyetheretherketone (PEEK) Wear Particles in Total Joint Replacement: A Systematic Review. Clin Orthop Relat Res 474, 2394–2404 (2016). https://doi.org/10.1007/s11999-016-4976-z
[3] Dusan Losic (2021): Advancing of titanium medical implants by surface engineering: recent progress and challenges, Expert Opinion on Drug Delivery, DOI: 10.1080/17425247.2021.1928071

















Comments